When an institution serves its own interest instead of the interests of its stakeholders, it’s not just a public policy problem -- people tend to get hurt. That’s why our government was designed to prevent conflicts of interest. We have a three-branch governmental system, with each branch keeping the others in check.
But regulatory agencies have gained unchecked power because corporate and non-governmental interests can create public policies that benefit their organizations with no regard for public interests.
We call this “the institutional imperative.”
This two-part article will introduce you to a child who has been severely injured during Pfizer’s COVID-19 vaccine trial. It will also demonstrate how the institutional imperative led to dangerously flawed decision-making by the FDA and other regulatory agencies.
Most importantly, you’ll learn what you can do to change this pattern of regulatory malfeasance.
Meet Maddie de Garay
Maddie de Garay volunteered to enter the Pfizer COVID-19 vaccine trials at Cincinnati Children's Hospital because she wanted to help save others from the virus. She’s fascinated by medicine and wants to be a nurse when she grows up.

Her mother, Stephanie, allowed both Maddie and her younger brother to enter the trial because she had friends who entered and suffered no ill effects. She trusted that her children would get the help needed to recover fully if side effects occurred. What happened instead was unspeakable.
Maddie’s first dose was typical. She had the fever, body aches and tiredness, but it cleared up in days, so Maddie’s mother had no fears when she took her to receive her second dose.
Maddie’s reaction to the second injection was very different. Less than 12 hours after, she was doubled over in pain. It was clear she was having a severe reaction, tormented by agonizing sensations of electrical shocks in her spine and wracked with nausea.
Maddie endured crushing chest pain that “felt like [her] heart was being ripped out of [her] neck,” EKG-diagnosed tachycardia, extreme dizziness, and cold, numb hands.
When Stephanie tried to report Maddie’s side effects, she learned that the app provided for any adverse event reports was shockingly limited.
This is very important.
A properly designed clinical trial must ensure that adverse event reporting isn’t biased by study administrators who have financial ties to the product. But the app provided as a supposedly unbiased adverse event reporting tool didn’t allow reporting reactions beyond a limited list.
Therefore, the study design was unequivocally flawed, and the public was not made aware of this deficiency before the vaccine mandates were invoked.
Photo by George Becker from Pexels
There was no unbiased way to report Maddie’s unusual symptoms.
The app provided to the de Garay family, called Trial Max, only logged reactions for seven days after each dose and would only let participants select from a limited list of expected side effects like fever, redness, pain and swelling.
Study administrators could have easily fixed this flaw by allowing a text box where participants could type in their symptoms, categorized as “other.” But according to the de Garay family, this was never provided as an option.
Therefore, the only way to report Maddie’s severe reaction was to call the biased study doctor or principal investigator. There was no unbiased way to report Maddie’s symptoms -- symptoms that are still plaguing her to this day.
The result? Pfizer’s report of Maddie’s severe adverse reactions was reduced to a mere five lines that claimed she was eventually diagnosed with “functional abdominal pain.” i.e., a stomach ache.

Maddie’s medical problems were lab-verified.
There is no reasonable excuse for the failure to report the full extent of Maddie’s injuries. Her severe adverse reactions were lab-verified on multiple occasions.
Maddie experienced over 35 adverse events by the data cutoff date of the trial, March 13, 2021.
These well-documented adverse events include:
- blood in her urine seven times and confirmed with a catheter-obtained sample,
- vision trouble,
- loss of feeling from her waist down,
- fainting,
- tremors,
- dizziness, and
- muscle weakness,
none of which were properly documented in the study data.
What other information was obscured in the COVID-19 vaccine trials?
If Maddie’s severe reaction was reduced to mere “functional abdominal pain,” what other severe reactions have been hidden from the public? Were there deaths? Were people who died or were incapacitated from the first dose removed from the trial?
When the FDA received a FOIA request to examine the data that was used to grant the EUA, the FDA asked for 75 years to release the data. Thankfully, they’ve been ordered to release the documents within eight months and Siri and Glimstad LLP have vowed to make these documents available to the general public on their website as they are released. But the FDA is pushing for delays yet again.
The aftermath
In the now over ten months since Maddie received the second shot, she lost her ability to walk, eats through an NG tube, has constant pain in her stomach, back, and neck, and can’t feel her legs. She had been to the ER 9 times and was hospitalized three times totaling 63 days by June 1st. In fact, Maddie was in the hospital when the EUA for ages 12 to 15 was approved.
Maddie’s experience has now come to light, and the general public is beginning to ask questions--as they should. It’s clear that Maddie’s case was underreported and the statistics offered to us don’t reflect her injuries.
How many other cases were swept under the rug before this drug was released, resulting in $93.5 million per day in liability-free profits for vaccine makers?
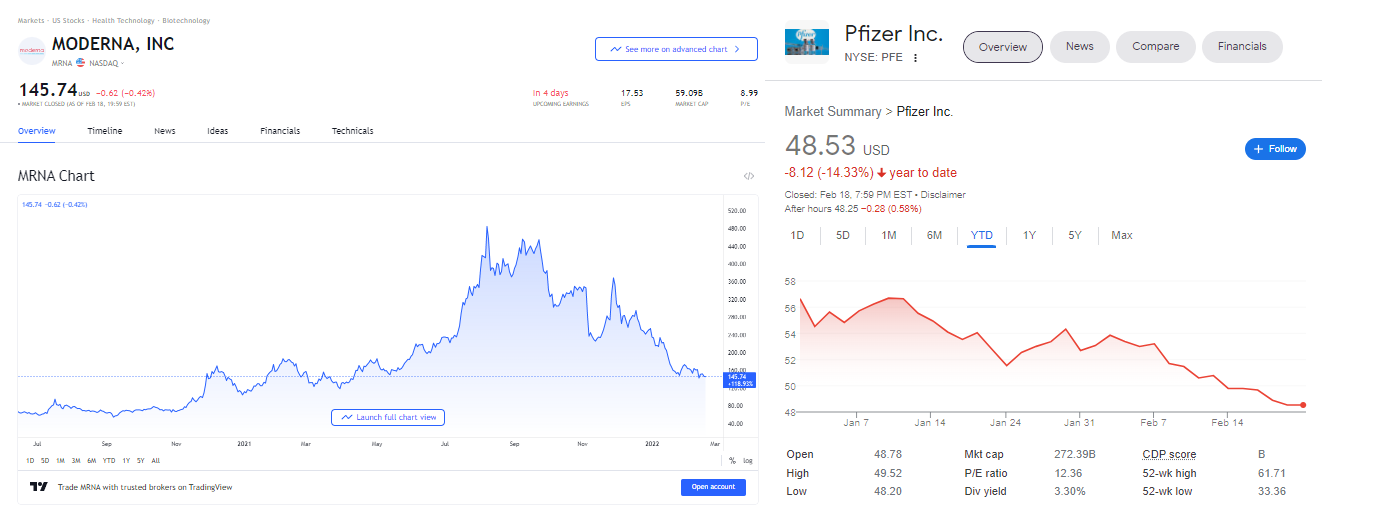
We should note that investors are asking this question too. Moderna’s stock has tanked over 70% from its high and Pfizer is on a steep decline. Perhaps investors caught wind that in contract law, fraud vitiates the contract.
When an institution becomes beholden to powers other than its stakeholders, this situation is known as “the institutional imperative.” Maddie’s case demonstrates how the institutional imperative presents a grave danger to public health and safety.
Were Maddie’s case properly reported, there would be at least 1 in 1,131 severe adverse events for the Pfizer trial of 12 to 15-year-olds. COVID was shown in a study of over 48,000 participants to have a zero percent fatality rate for children with no comorbid conditions.
Had Pfizer properly reported Maddie’s injuries, it would have been clear that the risks of the vaccine for healthy children aged 12 to 15 far outweigh the benefit. But the institutional imperative has a mind of its own.
There is a solution to this. The Constitution empowers us to hold a Convention of States to propose amendments that would permanently limit federal power and end the intra-industry cronyism that turns institutions like the FDA and CDC against our best interests.
Get involved by signing the petition at Convention of States. There are also volunteer positions available.






